Anthrax
Background and potential as a bioweapon
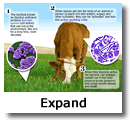 Anthrax is a serious infectious disease caused by gram-positive, rod-shaped bacteria known as Bacillus anthracis. It occurs naturally in soil and commonly affects domestic and wild animals worldwide. Anthrax can cause severe illness in both humans and animals (CDC, 2020).
Anthrax is a serious infectious disease caused by gram-positive, rod-shaped bacteria known as Bacillus anthracis. It occurs naturally in soil and commonly affects domestic and wild animals worldwide. Anthrax can cause severe illness in both humans and animals (CDC, 2020).
Anthrax is one of the oldest infectious diseases recorded in history. It is thought that descriptions of plagues in the Bible’s book of Exodus may have been outbreaks of anthrax in cattle and humans. Although anthrax spores distributed through the U.S. mail were responsible for several deaths in the fall of 2001, a large aerosol release of anthrax is a more significant threat to the U.S. population. A 1970, World Health Organization (WHO) report states "If a biological agent such as anthrax were used, an attack on a city by even a single bomber disseminating 50 kg of the dried agent in a suitable aerosol form would affect an area far in excess of 20 km2, with tens to hundreds of thousands of deaths" (WHO 1970).
Epidemiology
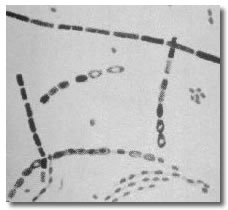 Anthrax is caused by infection with Bacillus anthracis, a gram-positive spore-forming rod. The spore form of anthrax can survive in the environment for many decades. Anthrax spores can be distributed in an aerosol form and are quite resistant to environmental degradation. Anthrax spores from 2 to 6 microns in size are the ideal size for infecting the mucosal surfaces of the lower respiratory tract. Manufacturing and distributing anthrax spores in this size range while avoiding the clumping of spore particles is one of the challenges facing bioterrorists attempting to use anthrax as a weapon of mass destruction. Both bacilli rods and spores are shown in this picture.
Anthrax is caused by infection with Bacillus anthracis, a gram-positive spore-forming rod. The spore form of anthrax can survive in the environment for many decades. Anthrax spores can be distributed in an aerosol form and are quite resistant to environmental degradation. Anthrax spores from 2 to 6 microns in size are the ideal size for infecting the mucosal surfaces of the lower respiratory tract. Manufacturing and distributing anthrax spores in this size range while avoiding the clumping of spore particles is one of the challenges facing bioterrorists attempting to use anthrax as a weapon of mass destruction. Both bacilli rods and spores are shown in this picture.
Human anthrax has three major clinical forms: cutaneous anthrax, inhalation anthrax, and gastrointestinal anthrax. Cutaneous anthrax occurs when the bacillus is introduced through the skin; inhalation anthrax through the respiratory tract, and gastrointestinal anthrax, by ingestion. Most anthrax cases are cutaneous forms of the disease.
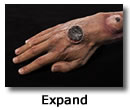 Cutaneous anthrax symptoms
Cutaneous anthrax symptoms
- Cutaneous anthrax is the most common form of anthrax infection, and the least dangerous.
- Anthrax spores enter subepidermal structures through a cut or scrape. This can happen when a person handles infected animals or contaminated animal products like wool, hides, or hai. The spores can also enter through the bite of infected flies.
- Resident macrophages phagocytize the spores; they germinate and mature into the vegetative B. anthrasis within the macrophage.
- The toxins produced by the vegetative B. anthrasis cause multiple cellular dysregulatory processes including disruption of water hemostasis leading to cytolysis and release of the bacteria from the macrophage.
- B.anthracis produce an anti-phagocytic capule that allows the bacteria to proliferate locally at the entry site. Anthrax toxins produced by the bacteria cause local edema, inflammation and necrotic lesions.
- B.anthracis can spread to the lymph nodes draining the wound, leading to toxemia, hemorrhage, sepsis, and organ failure.
- Signs of infection usually develops from 1 to 7 days after exposure.
- A group of small blisters or bumps that may itch
- Swelling can occur around the sore
- A painless skin sore (ulcer) with a black center that appears after the small blisters or bumps
- Most often the sore will be on the face, neck, arms, or hand
- Without treatment, up to 20% of people with cutaneous anthrax die. However, with proper treatment, almost all patients with cutaneous anthrax survive.
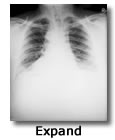 Inhalation anthrax
Inhalation anthrax
Inhalation of anthrax spores deposit the spores on the alveolar epithelium. Alveolar macrophages engulf and phagocytize the spores. The macrophages transport the spores via lymphatics to the mediastinal lymph nodes where they mature into vegetative bacilli, proliferate and release the components of anthrax Edema toxin and anthrax Lethal toxin. These toxins work synegistically to produce vascular dysfunction, progessive hypotension, hepatic and renal organ failure, hemorrhage, mediastinal, pleural, abdominal and pericardial edema (Sweeney et al. 2010)
The 2001 anthrax terrorist attack utilized highly refined anthrax spores. Anthrax spores were intentionally produced to maximize their pulmonary dispersion by reducing the size of the spore particles. The smaller the particles the greater the number and the deeper they can travel into the lungs. Models suggest that an aerosolized release of B. anthracis spores over a large urban population could result in a mass-casualty incident involving hundreds of thousands of illnesses and deaths. In a mass-casualty incident (i.e., cutaneous, gastrointestinal [GI], inhalation, and meningeal), inhalation anthrax and anthrax meningitis have the highest case-fatality rates.
Inhalation anthrax begins with flu-like symptoms (cough, fever, muscle aches). These symptoms may last two to three days, and then appear to go away for one or two days. Then the illness can come back, resulting in severe lung problems, difficulty breathing, and shock. Unless it’s treated, inhalation anthrax can be very dangerous – it’s fatal in up to 90 percent of cases. With treatment, during the anthrax attacks of 2001, the death rate was about 40 percent (Anthrax Facts n.d.).
Number and percentage of symptoms, signs, and diagnostic findings at hospital admission for patients in the United States with inhalation anthrax; 2001–2011
| Number and percentage of symptoms, signs, and diagnostic findings at hospital admission for patients in the United States with inhalation anthrax; 2001–2011 |
| Characteristic |
Number |
% |
Symptom |
|
|
Fever and chills
|
12/13 |
(92) |
Fatigue/Malaise
|
12/13 |
(92) |
Cough
|
11/13 |
(85) |
Nausea/Vomiting
|
9/13 |
(69) |
Diaphoresis
|
8/13 |
(62) |
Chest pain
|
7/13 |
(54) |
Myalgia
|
6/13 |
(46) |
Confusion
|
5/13 |
(38) |
Headache
|
4/13 |
(31) |
| Sign |
|
|
Heart rate >90 beats/min
|
13/13 |
(100) |
Abnormal core temperature fever >38.0°C or hypothermia <36.0°C)
|
8/13 |
(62) |
Hypoxemia (PaO2 <85 mm Hg)
|
3/9 |
(33) |
Tachypnea (>20 breaths/min)
|
2/13 |
(15) |
| Abnormal laboratory value |
|
|
Elevated transaminases (ALT or AST above normal limits)
|
9/11 |
(82) |
Leukocytosis (WBC >12 x 103/μL)
|
2/13 |
(15) |
| Radiographic finding |
|
|
Pleural effusion
|
9/13 |
(69) |
Infiltrate
|
8/13 |
(62) |
Mediastinal widening
|
5/13 |
(38) |
| Abbreviations: ALT = alanine transaminase; AST = aspartate transaminase; PaO2 = arterial partial pressure of oxygen; WBC = white blood cell. |
| Source: Clinical framework and medical countermeasure use during an anthrax ... (n.d.). Retrieved September 2, 2022, from https://www.cdc.gov/mmwr/pdf/rr/rr6404.pdf |
Gastrointestinal anthrax symptoms
Gastrointestinal anthrax has rarely been reported in the United States. Symptoms usually develop from 1 to 7 days after exposure. ingestion of raw or undercooked meat from an animal infected with anthrax, can transmit gastrointestinal anthrax. Once ingested, anthrax spores can affect the upper gastrointestinal tract (throat and esophagus), stomach, and intestines, causing a wide variety of symptoms. Without treatment, more than half of patients with gastrointestinal anthrax die. However, with proper treatment, 60% of patients survive.
- Fever and chills
- Swelling of neck or neck glands
- Sore throat
- Painful swallowing
- Hoarseness
- Nausea and vomiting, especially bloody vomiting
|
- Diarrhea or bloody diarrhea
- Headache
- Flushing (red face) and red eyes
- Stomach pain
- Fainting
- Swelling of abdomen (stomach)
|
Injection anthrax symptoms
Recently, another type of anthrax infection has been identified in heroin-injecting drug users in northern Europe. Symptoms may be similar to those of cutaneous anthrax, but there may be infection deep under the skin or in the muscle where the drug was injected. Injection anthrax can spread throughout the body faster and be harder to recognize and treat. Lots of other more common bacteria can cause skin and injection site infections, so a skin or injection site infection in a drug user does not necessarily mean the person has anthrax.
• Fever and chills
• A group of small blisters or bumps that may itch, appearing where the drug was injected
• A painless skin sore with a black center that appears after the blisters or bumps
• Swelling around the sore
• Abscesses deep under the skin or in the muscle where the drug was injected
Pathogenesis
Inactive B. anthracis spores can enter the body through 1) abraded skin, 2) ingested with food or drink, 3) inhaled into the bronchi and lung alveoli, and 4) injected with contaminated illicit drugs.
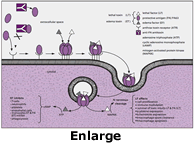 When spores encounter an aqueous environment containing appropriate nutrients, they can germinate and grow as vegetative cells (Chesnokova, 2009). Vegetative B. anthracis cells produce three protein factors that individually are non-toxic. However, when the proteins are joined together and translocated into the target cell, they become cytotoxic. The anthrax toxins are composed of a protein complex of Protective Antigen (PA) and Edema Factor (EF) or a PA and a Lethal Factor (LF). The PA portion attaches to an anthrax toxin receptor, (ANTXR1, AKA - Tumour Endothelial Marker 8) or (ANTXR2, AKA - Capillary Morphogenesis Gene 2 (CMG2)), both are highly expressed on the surface membrane of epithelial cells (particularly in the vasculature, respiratory epithelium, intestines, and keratinocytes. PA can complex with EF or LF. When a protein complex of PA+EF or PA+LF is bound to ANTXR1 or ANTXR2, endocytosis is induced, bringing Edema toxin or Lethal toxin into the cytosol (Avril, 2022).
When spores encounter an aqueous environment containing appropriate nutrients, they can germinate and grow as vegetative cells (Chesnokova, 2009). Vegetative B. anthracis cells produce three protein factors that individually are non-toxic. However, when the proteins are joined together and translocated into the target cell, they become cytotoxic. The anthrax toxins are composed of a protein complex of Protective Antigen (PA) and Edema Factor (EF) or a PA and a Lethal Factor (LF). The PA portion attaches to an anthrax toxin receptor, (ANTXR1, AKA - Tumour Endothelial Marker 8) or (ANTXR2, AKA - Capillary Morphogenesis Gene 2 (CMG2)), both are highly expressed on the surface membrane of epithelial cells (particularly in the vasculature, respiratory epithelium, intestines, and keratinocytes. PA can complex with EF or LF. When a protein complex of PA+EF or PA+LF is bound to ANTXR1 or ANTXR2, endocytosis is induced, bringing Edema toxin or Lethal toxin into the cytosol (Avril, 2022).
- Lethal factor (LF) has been shown by Kirby to induce endothelial apoptosis and that Lethal toxin affects microvessel and large vessel endothelial cells causing the vascular pathology and hemorrhage during systemic anthrax (Kirby, 2004). Park, et al., demonstrated that Lethal toxin causes the death of macrophages which allows B. anthracis to avoid detection by the innate immune system.
- Edema factor (EF) is an adenylate cyclase that converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (c-AMP). Excess c-AMP disrupts endothelial cytoskeletal integrity leading to paracellular permeability and the transient formation of large transcellular tunnels that permit fluid and bacilli to cross the endothelial barrier causing the edema and septic shock-like manifestations that are the hallmarks of infection by B. anthracis (Maddugoda et al., 2011).
Autopsy finding from persons who died from inhalation anthrax in the 2001 anthrax attack had extensive amounts of serosanguinous fluid in pleural cavities and edema and hemorrhage of the mediastinum and surrounding soft tissues, and 48% had cerebral edema, 21% had ascites, 17% had pericardial effusions, and 14% had petechial rash. Mediastinal lymph nodes and spleen also showed hemorrhage and necrosis (Hendricks 2014).
Clinical course
Symptoms of inhalational anthrax generally occur after an incubation period ranging between 1 and 6 days. After the incubation period, a nonspecific flu like illness occurs, with symptoms of fever, myalgia or muscle aches, headache, a non-productive cough, and mild chest discomfort. Often, a brief intervening period of improvement follows the initial symptoms and rapid deterioration. High fever, difficulty breathing, cyanosis, and shock characterize this next phase. Death is universal in untreated cases and may occur in as many as 100% of treated cases if therapy is started more than 48 hours after symptoms begin. A large number of gram-positive bacilli in nasal swabs or in environmental samples can help confirm a diagnosis of anthrax where intentional release is suspected.
Treatment
Anthrax Vaccine (CDC 2016)
Pre-exposure Vaccination
CDC recommends anthrax vaccination for three groups of adults 18 through 65 years of age who may be at risk for occupational exposure to the bacteria:
- Certain laboratory workers who work with anthrax
- Some people who handle animals or animal products, such as veterinarians who handle infected animals
- Certain U.S. military personnel
Post-exposure Vaccination
CDC recommends a post-exposure regimen of 60 days of appropriate antimicrobial prophylaxis combined with 3 subcutaneous doses of the anthrax vaccine for previously unvaccinated people 18 years or older who have been exposed to aerosolized Bacillus anthracis spores.
Contraindications and Precautions
Anthrax vaccine should not be administered to:
- A person who has ever had a severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a vaccine component
- Pregnant women when the risk to anthrax exposure is low
Anthrax vaccine may be administered, if the provider deems the benefits of vaccination to outweigh the risks (CDC 2016).
CDC Antibiotic Emergency Use Instructions
Antitoxin
The U.S. government stockpiles antibody-based antitoxins for the treatment of adult and pediatric patients with inhalation anthrax for use in combination with appropriate antimicrobial therapy. These antitoxins bind to PA with high affinity in a dose-dependent manner. CDC’s Strategic National Stockpile contains monoclonal and polyclonal antitoxins. Although polyclonal antitoxins bind PA at multiple sites, and bind other antigens besides PA, available data do not provide enough evidence to preferentially recommend one antitoxin over another (MMWR 2015).
Instant Feedback:
Ciprofloxacin and doxycycline can ________ the half-life of caffeine.
References
Anthrax facts (fact sheet) - Minnesota Dept. of Health. (n.d.). Retrieved September 2, 2022, from https://www.health.state.mn.us/diseases/anthrax/anthrax.html
Anthrax in Humans and Animals. 4th edition. Geneva: World Health Organization; 2008. 2, Etiology and ecology. Available from: https://www.ncbi.nlm.nih.gov/books/NBK310478/
Avril, A., Tournier, J.-N., Paucod, J.-C., Fournes, B., Thullier, P., & Pelat, T. (2022). Antibodies against anthrax toxins: A long way from Benchlab to the bedside. Toxins, 14(3), 172. https://doi.org/10.3390/toxins14030172
CDC. (2016). Anthrax vaccination recommendations. Centers for Disease Control and Prevention. Retrieved August 14, 2022, from https://www.cdc.gov/vaccines/vpd/anthrax/hcp/recommendations.html
CDC. (2020) Anthrax. Anthrax as a bioterrorism weapon. Retrieved September 2, 2022, from https://www.cdc.gov/anthrax/bioterrorism/index.html
Chesnokova, O. N., McPherson, S. A., Steichen, C. T., & Turnbough, C. L. (2009). The spore-specific alanine racemase of bacillus anthracis and its role in suppressing germination during spore development. Journal of Bacteriology, 191(4), 1303–1310. https://doi.org/10.1128/jb.01098-08
FDA. Vaccines. Anthrax (2018). Retrieved 8/13/2022 from: Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis [Internet]. 2014 Feb [date cited]. http://dx.doi.org/10.3201/eid2002.130687
Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis [Internet]. 2014 Feb [date cited]. http://dx.doi.org/10.3201/eid2002.130687
Kirby J. E. (2004). Anthrax lethal toxin induces human endothelial cell apoptosis. Infection and immunity, 72(1), 430–439. https://doi.org/10.1128/IAI.72.1.430-439.2004
Maddugoda, M. P., Stefani, C., Gonzalez-Rodriguez, D., Saarikangas, J., Torrino, S., Janel, S., Munro, P., Doye, A., Prodon, F., Aurrand-Lions, M., Goossens, P. L., Lafont, F., Bassereau, P., Lappalainen, P., Brochard, F., & Lemichez, E. (2011). Camp signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via ARP2/3-driven actin polymerization. Cell Host & Microbe, 10(5), 464–474. https://doi.org/10.1016/j.chom.2011.09.014
MMWR (2015) Clinical Framework and Medical Countermeasure Use During an Anthrax Mass-Casualty Incident CDC Recommendations. Retrieved 9/2/2022 from https://www.cdc.gov/mmwr/pdf/rr/rr6404.pdf
Park, J. M., Greten, F. R., Li, Z. W., & Karin, M. (2002). Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science (New York, N.Y.), 297(5589), 2048–2051. https://doi.org/10.1126/science.1073163
Sweeney, D. A., Cui, X., Solomon, S. B., Vitberg, D. A., Migone, T. S., Scher, D., Danner, R. L., Natanson, C., Subramanian, G. M., & Eichacker, P. Q. (2010). Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. The Journal of infectious diseases, 202(12), 1885–1896. https://doi.org/10.1086/657408
World Health Organization. (1970). HEALTH ASPECTS OF CHEMICAL AND BIOLOGICAL WEAPONS. Retrieved September 2, 2022, from https://iris.wpro.who.int/?sequence=1
 Anthrax is a serious infectious disease caused by gram-positive, rod-shaped bacteria known as Bacillus anthracis. It occurs naturally in soil and commonly affects domestic and wild animals worldwide. Anthrax can cause severe illness in both humans and animals (CDC, 2020).
Anthrax is a serious infectious disease caused by gram-positive, rod-shaped bacteria known as Bacillus anthracis. It occurs naturally in soil and commonly affects domestic and wild animals worldwide. Anthrax can cause severe illness in both humans and animals (CDC, 2020). Anthrax is caused by infection with Bacillus anthracis, a gram-positive spore-forming rod. The spore form of anthrax can survive in the environment for many decades. Anthrax spores can be distributed in an aerosol form and are quite resistant to environmental degradation. Anthrax spores from 2 to 6 microns in size are the ideal size for infecting the mucosal surfaces of the lower respiratory tract. Manufacturing and distributing anthrax spores in this size range while avoiding the clumping of spore particles is one of the challenges facing bioterrorists attempting to use anthrax as a weapon of mass destruction. Both bacilli rods and spores are shown in this picture.
Anthrax is caused by infection with Bacillus anthracis, a gram-positive spore-forming rod. The spore form of anthrax can survive in the environment for many decades. Anthrax spores can be distributed in an aerosol form and are quite resistant to environmental degradation. Anthrax spores from 2 to 6 microns in size are the ideal size for infecting the mucosal surfaces of the lower respiratory tract. Manufacturing and distributing anthrax spores in this size range while avoiding the clumping of spore particles is one of the challenges facing bioterrorists attempting to use anthrax as a weapon of mass destruction. Both bacilli rods and spores are shown in this picture.

